pH Calculator
With this pH calculator, you can determine the pH of a solution in a few ways. It can convert pH to H+, as well as calculate pH from the ionization constant and concentration. The pH value is an essential factor in chemistry, medicine, and daily life. Read the text below to find out what is the pH scale and the pH formula. In the end, we will also explain how to calculate pH with an easy step-by-step solution.
Our calculator may ask you for the concentration of the solution. If you don't know, you can calculate it using our concentration calculator. You can also use the solution dilution calculator to calculate the concentration of ions in a diluted solution.
The pH scale
The pH scale (pH) is a numeric scale used to define how acidic or basic an aqueous solution is. It commonly ranges between 0 and 14 but can go beyond these values if sufficiently acidic/basic. The pH value is logarithmically and is inversely related to the concentration of hydrogen ions in a solution. The pH to H+ formula that represents this relation is:
The solution is acidic if its pH is less than 7. If the pH is higher, the solution is basic (also referred to as alkaline). Solutions with a pH that is equal to 7 are neutral.
Apart from the mathematical way of determining pH, you can also use pH indicators. The most universally used pH test is the litmus paper. It changes its color according to the pH of the solution in which it was dipped. These colors often inspire colorful pH scales:
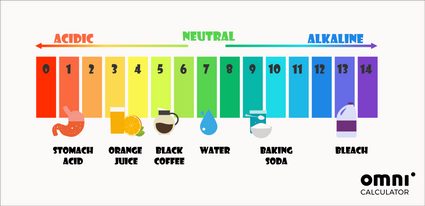
The pH in our bodies is close to neutral. For example, the pH of blood should be around 7.4. The only exception is the stomach, where stomach acids can even reach a pH of 1.
Molecules can have a pH at which they are free of a negative charge. That is what our isoelectric point calculator determines.
Definitions of an acid and a base
Three different theories define acid and base:
- According to the Arrhenius theory, in an aqueous solution, an acid is a substance able to donate hydrogen ions, while a base donates hydroxide ions.
- Brønsted–Lowry theory says that acid can donate protons while a base can accept them.
- Lewis theory states that an acid is something that can accept electron pairs. Analogously, a base donates electron pairs.
The higher the concentration of hydrogen ions from acid molecules, the lower the pH of the solution and, consequently, the higher its acidity. The reverse is true for hydroxide ions and bases. The higher the concentration of hydroxide ions from base molecules, the higher the pH of the solution and, consequently, the higher its basicity.
We can describe the reaction of an acid, HA, in water as:
with the acid ionization constant:
A similar chemical reaction between base BOH and water looks like this:
The next equation gives the base ionization constant for the above formula:
If you want to know more about chemical equilibrium constants, check out the equilibrium constant calculator or the reaction quotient calculator.
How to find pH – pH formula
PH is defined as the negative of the base-ten logarithm of the molar concentration of hydrogen ions present in the solution. The unit for the concentration of hydrogen ions is moles per liter. To determine pH, you can use this pH to H⁺ formula:
If you already know pH but want to calculate the concentration of ions, use this transformed pH equation:
There also exists a pOH scale - which is less popular than the pH scale. pOH is the negative of the logarithm of the hydroxide ion concentration:
or:
pH and pOH are related to one another by this pOH and pH equation:
How to calculate pH? – step by step solution
Here are the steps to calculate the pH of a solution:
-
Let's assume that the concentration of hydrogen ions is equal to 0.0001 mol/L.
-
Calculate pH by using the pH to H⁺ formula:
- Now, you can also easily determine pOH and a concentration of hydroxide ions using the formulas:
Of course, you don't have to perform all of these calculations by hand! Choose the option to determine pH with ion concentration in the calculator, and type in any of these four values! Then, watch as the tool does all the work for you!
- Alternatively, you can find a chemical from the lists (of acids or bases). Let's say you want to know how to find the pH of formic acid –
HCOOH. ItsKais0.00018. - Choose the concentration of the chemical. Let's assume that it's equal to
0.1 mol/L. - To find a concentration of H⁺ ions, you have to...:
where:
Here is the molar concentration of the solution, and is equal to the molar concentration of H⁺.
For 0.1 M HCOOH:
Now you know how to calculate pH using pH equations. If you find these calculations time-consuming, feel free to use our pH calculator. Select your chemical and its concentration, and watch it do all the work for you.
FAQ
What is pH?
pH measures the concentration of positive hydrogen ions in a solution. This quantity is correlated to the acidity of a solution: the higher the concentration of hydrogen ions, the lower the pH. This correlation derives from the tendency of an acidic substance to cause dissociation of water: the higher the dissociation, the higher the acidity.
How do I calculate the pH of a solution?
To calculate the pH of a solution:
-
Measure the concentration of hydrogen ion in the solution. Alternatively, you can measure the activity of the same species. We can call it
[H+]. -
Calculate the base 10 logarithm of this quantity:
log10([H+]). -
Take the additive inverse of this quantity. The pH is given by:
pH = - log10([H+])
What is the difference between pH and pOH?
The pOH is a similar measurement to the pH and correlates to the concentration of hydroxide ions in a solution. The formula for the pOH is:
pOH = -log10([OH-])
In specific conditions (aqueous solutions at room temperature), we can define a useful relationship between pH and pOH:
pH = 14 - pOH
What are some examples of pH?
Here are some examples of pH:
-
The pH of pure water is
7, which is the midpoint of the pH scale. -
The pH of our stomach varies from
1.5to3.5: our stomach is quite acidic! -
The acid in your car's battery has a pH of about
0.5: don't put your hands in there! -
House products like drain cleaners are strong bases: some can reach a pH of
14!